Auto-Immune Diseases – Pipeline Products by Stage of Development 23
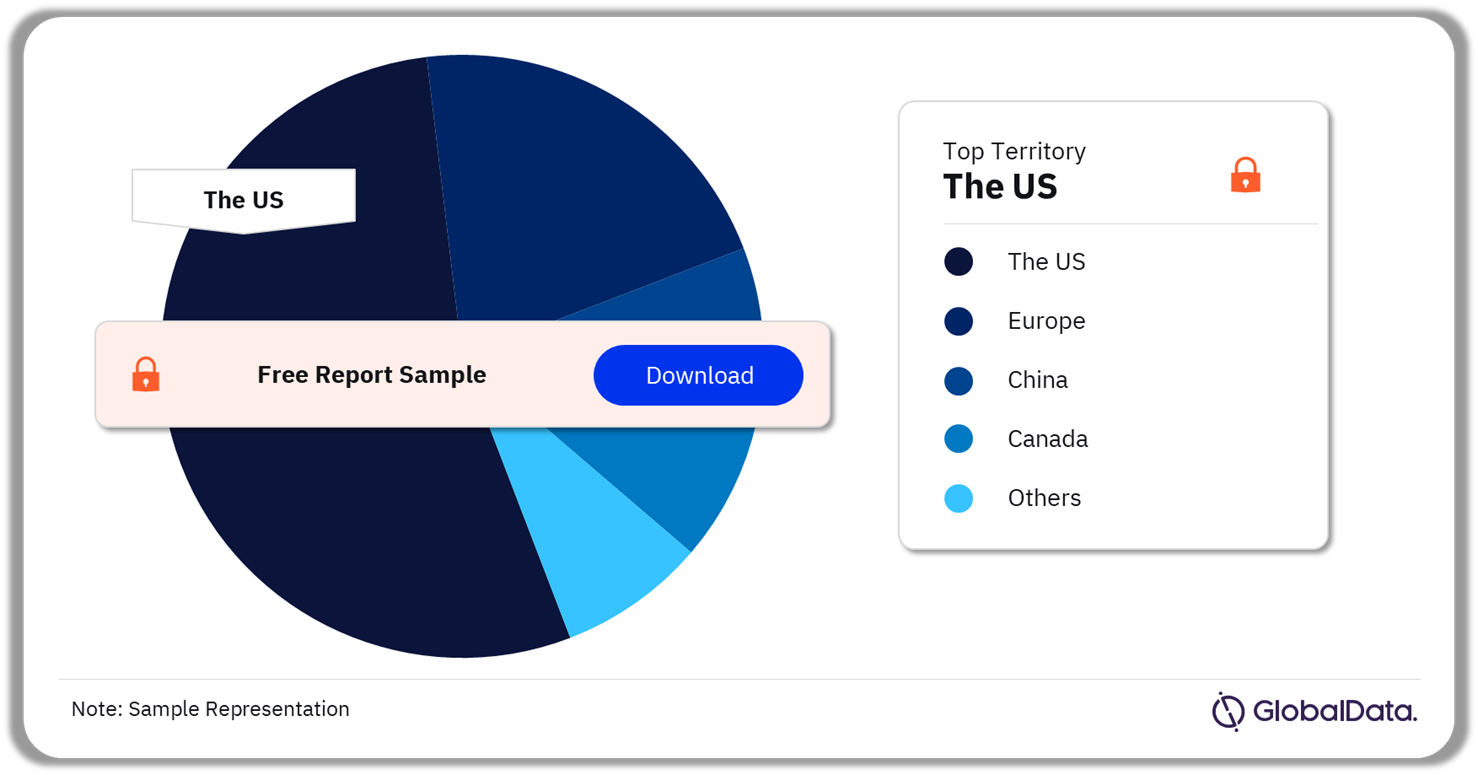
Auto-Immune Diseases – Pipeline Products by Territory 24
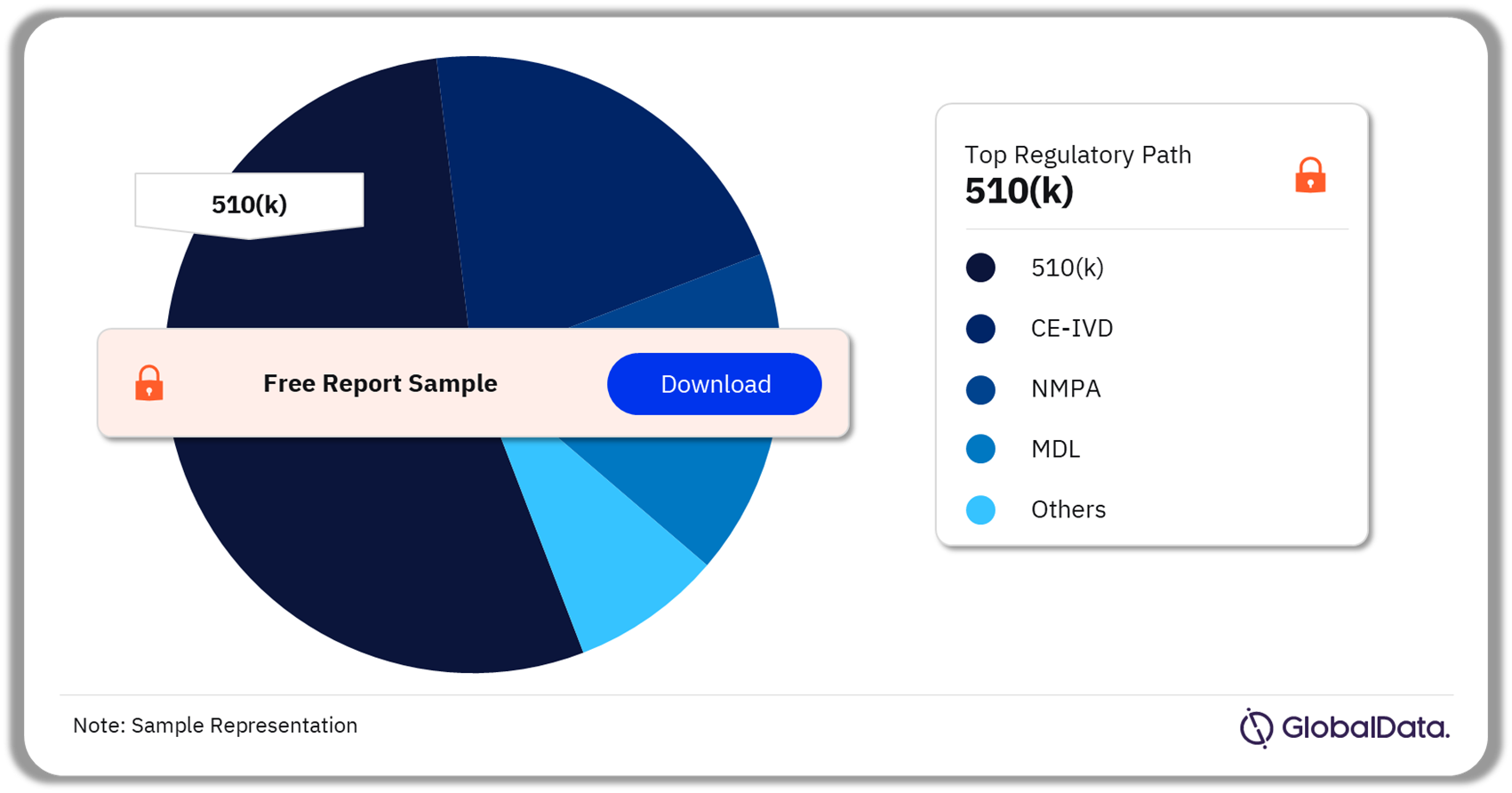
Auto-Immune Diseases – Pipeline Products by Regulatory Path 25
Auto-Immune Diseases – Pipeline Products by Estimated Approval Date 26
Auto-Immune Diseases – Ongoing Clinical Trials 27
Auto-Immune Diseases Companies – Pipeline Products by Stage of Development 28
Auto-Immune Diseases – Pipeline Products by Stage of Development 33
Aarhus University Pipeline Products & Ongoing Clinical Trials Overview 39
Biomarker Test – Autoimmune Diseases – Product Status 39
Biomarker Test – Autoimmune Diseases – Product Description 39
Biomarker Test – Multiple Sclerosis – Product Status 40
Biomarker Test – Multiple Sclerosis – Product Description 40
Adaptive Biotechnologies Corp Pipeline Products & Ongoing Clinical Trials Overview 41
Non-Invasive Diagnostic Test – Celiac Disease – Product Status 41
Non-Invasive Diagnostic Test – Celiac Disease – Product Description 41
T-Detect – Multiple Sclerosis – Product Status 42
T-Detect – Multiple Sclerosis – Product Description 42
T-Detect – Rheumatoid Arthritis – Product Status 42
T-Detect – Rheumatoid Arthritis – Product Description 43
T-Detect – Ulcerative Colitis – Product Status 43
T-Detect – Ulcerative Colitis – Product Description 43
Aditxt Inc Pipeline Products & Ongoing Clinical Trials Overview 44
AditxtScore – Autoimmune Diseases – Product Status 44
AditxtScore – Autoimmune Diseases – Product Description 44
Albert Ludwigs University of Freiburg Pipeline Products & Ongoing Clinical Trials Overview 45
Biomarker Based Assay – Systemic Sclerosis – Product Status 45
Biomarker Based Assay – Systemic Sclerosis – Product Description 45
Alere Inc Pipeline Products & Ongoing Clinical Trials Overview 46
AIMS System – Cardiolipin IgG Test – Product Status 46
AIMS System – Cardiolipin IgG Test – Product Description 46
AtheNA Multi-Lyte System – Cardiolipin IgG Test – Product Status 47
AtheNA Multi-Lyte System – Cardiolipin IgG Test – Product Description 47
Amarantus Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 48
Diagnostic Assay – Neuromyelitis Optica – Product Status 48
Diagnostic Assay – Neuromyelitis Optica – Product Description 48
Diagnostic Assay – Paraneoplastic Disease – Product Status 49
Diagnostic Assay – Paraneoplastic Disease – Product Description 49
apDia nv Pipeline Products & Ongoing Clinical Trials Overview 50
Diagnostic Test – MS (Multiple Sclerosis) – Product Status 50
Diagnostic Test – MS (Multiple Sclerosis) – Product Description 50
Asylia Diagnostics BV Pipeline Products & Ongoing Clinical Trials Overview 51
CDx Assay – Systemic Lupus Erythematosus – Product Status 51
CDx Assay – Systemic Lupus Erythematosus – Product Description 51
CDx Assay – Systemic Sclerosis (SS) – Product Status 52
CDx Assay – Systemic Sclerosis (SS) – Product Description 52
Autoimmune Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 53
Anti-Squalene Antibody Assay – Gulf War Syndrome – Product Status 53
Anti-Squalene Antibody Assay – Gulf War Syndrome – Product Description 53
Autoimmune Disease Virus Assay – Grave’s Disease – Product Status 54
Autoimmune Disease Virus Assay – Grave’s Disease – Product Description 54
Autoimmune Disease Virus Assay – Juvenile Rheumatoid Arthritis – Product Status 54
Autoimmune Disease Virus Assay – Juvenile Rheumatoid Arthritis – Product Description 55
Autoimmune Disease Virus Assay – Sjogren Syndrome – Product Status 55
Autoimmune Disease Virus Assay – Sjogren Syndrome – Product Description 55
Autoimmune Disease Virus Assay – Systemic Lupus Erythematosus – Product Status 56
Autoimmune Disease Virus Assay – Systemic Lupus Erythematosus – Product Description 56
Avotres Inc Pipeline Products & Ongoing Clinical Trials Overview 57
AVT-Dx001 – Product Status 57
AVT-Dx001 – Product Description 57
Beckman Coulter Inc Pipeline Products & Ongoing Clinical Trials Overview 58
SYNCHRON LXi 725 Anti-dsDNA Assay – Product Status 58
SYNCHRON LXi 725 Anti-dsDNA Assay – Product Description 58
Bellvitge Biomedical Research Institute Pipeline Products & Ongoing Clinical Trials Overview 59
Diagnostic Device – Autoimmune Encephalitis – Product Status 59
Diagnostic Device – Autoimmune Encephalitis – Product Description 59
BG Medicine Inc Pipeline Products & Ongoing Clinical Trials Overview 60
Diagnostic Assay – Multiple Sclerosis – Product Status 60
Diagnostic Assay – Multiple Sclerosis – Product Description 60
Biogen Inc Pipeline Products & Ongoing Clinical Trials Overview 61
Companion Diagnostic Test – Sjogren’s Syndrome – Product Status 61
Companion Diagnostic Test – Sjogren’s Syndrome – Product Description 61
BIOHOPE Scientific SL Pipeline Products & Ongoing Clinical Trials Overview 62
Immunobiogram – Lupus – Product Status 62
Immunobiogram – Lupus – Product Description 62
Immunobiogram – Psoriasis – Product Status 63
Immunobiogram – Psoriasis – Product Description 63
Biomedal SL Pipeline Products & Ongoing Clinical Trials Overview 64
ELISA DELIAC GFD – Product Status 64
ELISA DELIAC GFD – Product Description 64
Stick DELIAC GFD – Product Status 65
Stick DELIAC GFD – Product Description 65
Bionure Farma SL (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 66
BN – 301 Diagnostic/Prognostic Test – Product Status 66
BN – 301 Diagnostic/Prognostic Test – Product Description 66
BioShai Ltd Pipeline Products & Ongoing Clinical Trials Overview 67
PDx Prognostic Test – Product Status 67
PDx Prognostic Test – Product Description 67
PDx Screening Test – Product Status 68
PDx Screening Test – Product Description 68
PDx Test – Product Status 68
PDx Test – Product Description 69
Biotome Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 70
Biomarker-Based Diagnostic Test – Acute Rheumatic Fever – Product Status 70
Biomarker-Based Diagnostic Test – Acute Rheumatic Fever – Product Description 70
CDI Laboratories Inc Pipeline Products & Ongoing Clinical Trials Overview 71
Diagnostic Test – Systemic Lupus Erythematosus – Product Status 71
Diagnostic Test – Systemic Lupus Erythematosus – Product Description 71
Celdara Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 72
Diagnostic Assay – Diabetes – Product Status 72
Diagnostic Assay – Diabetes – Product Description 72
Charite University Hospital of Berlin Pipeline Products & Ongoing Clinical Trials Overview 73
Diagnostic Test – Celiac Disease – Product Status 73
Diagnostic Test – Celiac Disease – Product Description 73
Chronix Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 74
Companion Diagnostic Test – Multiple Sclerosis – Product Status 74
Companion Diagnostic Test – Multiple Sclerosis – Product Description 74
Diagnostic Test – Systemic Lupus Erythematous – Product Status 75
Diagnostic Test – Systemic Lupus Erythematous – Product Description 75
Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 76
Biomarker Panel – Lupus Nephritis – Product Status 76
Biomarker Panel – Lupus Nephritis – Product Description 76
Cizzle Biotechnology Holdings Plc Pipeline Products & Ongoing Clinical Trials Overview 77
Companion Diagnostic Test – Autoimmune Disease – Product Status 77
Companion Diagnostic Test – Autoimmune Disease – Product Description 77
Co-Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 78
Diagnostic Test – Auto-immune Disorder – Product Status 78
Diagnostic Test – Auto-immune Disorder – Product Description 78
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 79
Diagnostic Assay- NCGS/NCWS – Product Status 79
Diagnostic Assay- NCGS/NCWS – Product Description 79
Crescendo Bioscience Inc Pipeline Products & Ongoing Clinical Trials Overview 80
Multi-Biomarker Test – Ankylosing Spondylitis – Product Status 80
Multi-Biomarker Test – Ankylosing Spondylitis – Product Description 80
Multi-Biomarker Test – Autoimmune Diseases – Product Status 81
Multi-Biomarker Test – Autoimmune Diseases – Product Description 81
Multi-Biomarker Test – Psoriatic Arthritis – Product Status 81
Multi-Biomarker Test – Psoriatic Arthritis – Product Description 82
Multi-Biomarker Test – Systemic Lupus Erythematosus – Product Status 82
Multi-Biomarker Test – Systemic Lupus Erythematosus – Product Description 82
Department of Biomedical Engineering Columbia University Pipeline Products & Ongoing Clinical Trials Overview 83
AutoDetect – Autoimmune Lupus Mediated Myocarditis – Product Status 83
AutoDetect – Autoimmune Lupus Mediated Myocarditis – Product Description 83
Digna Biotech SL Pipeline Products & Ongoing Clinical Trials Overview 84
Anti-CD-137 Antibodies Assay – Product Status 84
Anti-CD-137 Antibodies Assay – Product Description 84
Prognostic Test – Multiple Sclerosis – Product Status 85
Prognostic Test – Multiple Sclerosis – Product Description 85
DNAlytics SA Pipeline Products & Ongoing Clinical Trials Overview 86
RheumaKit-Tx – Product Status 86
RheumaKit-Tx – Product Description 86
Enterome Bioscience SA Pipeline Products & Ongoing Clinical Trials Overview 87
Diagnostic Test – Multiple Sclerosis – Product Status 87
Diagnostic Test – Multiple Sclerosis – Product Description 87
Diagnostic Test – Type 2 Diabetes – Product Status 88
Diagnostic Test – Type 2 Diabetes – Product Description 88
Exagen Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 89
AVISE RA Dx – Product Status 89
AVISE RA Dx – Product Description 89
AVISE RA Monitoring Device – Product Status 90
AVISE RA Monitoring Device – Product Description 90
AVISE RA Prognostic Device – Product Status 90
AVISE RA Prognostic Device – Product Description 91
AVISE SLE Monitor Plus – Product Status 91
AVISE SLE Monitor Plus – Product Description 91
Exodus Biosciences LLC Pipeline Products & Ongoing Clinical Trials Overview 92
AIMS – Product Status 92
AIMS – Product Description 92
Ezose Sciences Inc. Pipeline Products & Ongoing Clinical Trials Overview 93
Biomarker Diagnostic Assay – Multiple Sclerosis – Product Status 93
Biomarker Diagnostic Assay – Multiple Sclerosis – Product Description 93
Flamentera AG Pipeline Products & Ongoing Clinical Trials Overview 94
Diagnostic Test – Celiac Disease – Product Status 94
Diagnostic Test – Celiac Disease – Product Description 94
Genalyte Inc Pipeline Products & Ongoing Clinical Trials Overview 95
Maverick ANA 11 Assay Kit – Product Status 95
Maverick ANA 11 Assay Kit – Product Description 95
GeNeuro SA Pipeline Products & Ongoing Clinical Trials Overview 96
MSRV-Env Based Diagnostic Test – Multiple Sclerosis – Product Status 96
MSRV-Env Based Diagnostic Test – Multiple Sclerosis – Product Description 96
Grifols SA Pipeline Products & Ongoing Clinical Trials Overview 97
Diagnostic Test – Autoimmune Diseases – Product Status 97
Diagnostic Test – Autoimmune Diseases – Product Description 97
Guangzhou Wondfo Biotech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 98
Finecare Diagnostic Assay – Anti-CCP – Product Status 98
Finecare Diagnostic Assay – Anti-CCP – Product Description 99
Finecare Diagnostic Assay – RF – Product Status 99
Finecare Diagnostic Assay – RF – Product Description 99
Hannover Medical School Pipeline Products & Ongoing Clinical Trials Overview 100
Diagnostic Test – Multiple Sclerosis – Product Status 100
Diagnostic Test – Multiple Sclerosis – Product Description 100
HealthTell Inc Pipeline Products & Ongoing Clinical Trials Overview 101
ImmunoSignature Test – Dermatomyositis – Product Status 101
ImmunoSignature Test – Dermatomyositis – Product Description 101
ImmunoSignature Test – Multiple Sclerosis – Product Status 102
ImmunoSignature Test – Multiple Sclerosis – Product Description 102
ImmunoSignature Test – Rheumatoid Arthritis – Product Status 102
ImmunoSignature Test – Rheumatoid Arthritis – Product Description 103
ImmunoSignature Test – Scleroderma – Product Status 103
ImmunoSignature Test – Scleroderma – Product Description 103
ImmunoSignature Test – Sjogren’s Syndrome – Product Status 104
ImmunoSignature Test – Sjogren’s Syndrome – Product Description 104
ImmunoSignature Test – SLE Differential Dx – Product Status 104
ImmunoSignature Test – SLE Differential Dx – Product Description 105
ImmunoSignature Test – SLE-Disease Activty/Flare – Product Status 105
ImmunoSignature Test – SLE-Disease Activty/Flare – Product Description 105
ImmunoSignature Test – Ulcerative Colitis – Product Status 106
ImmunoSignature Test – Ulcerative Colitis – Product Description 106
Helmholtz Centre for Infection Research Pipeline Products & Ongoing Clinical Trials Overview 107
ELISA Based Assay – Rheumatic Fever – Product Status 107
ELISA Based Assay – Rheumatic Fever – Product Description 107
HOB Biotech Group Suzhou Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 108
HOB Biotech Diagnostic Assay – Auto-immunity – Product Status 108
HOB Biotech Diagnostic Assay – Auto-immunity – Product Description 108
Hospital for Special Surgery Pipeline Products & Ongoing Clinical Trials Overview 109
Biomarker Test – Systemic Lupus Erythematosus – Product Status 109
Biomarker Test – Systemic Lupus Erythematosus – Product Description 109
HTG Molecular Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 110
NGS-Based Theranostic Tool – Product Status 110
NGS-Based Theranostic Tool – Product Description 110
Hycor Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 111
HYTEC 288 PLUS – Centromere Test – Product Status 111
HYTEC 288 PLUS – Centromere Test – Product Description 111
HYTEC 288 PLUS – ANCA Profile Test – Product Status 112
HYTEC 288 PLUS – ANCA Profile Test – Product Description 112
Ignyta Inc Pipeline Products & Ongoing Clinical Trials Overview 113
TrailblazeDx Test – Systemic Lupus Erythemetosus – Product Status 113
TrailblazeDx Test – Systemic Lupus Erythemetosus – Product Description 113
TrailblazeTx Test – SLE (Systemic Lupus Erythemetosus) – Product Status 114
TrailblazeTx Test – SLE (Systemic Lupus Erythemetosus) – Product Description 114
Immunarray Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 115
Diagnostic Test – Multiple Sclerosis – Product Status 115
Diagnostic Test – Multiple Sclerosis – Product Description 115
Immunovia AB Pipeline Products & Ongoing Clinical Trials Overview 116
IMMray Primary Sjogren’s Syndrome Assay – Product Status 116
IMMray Primary Sjogren’s Syndrome Assay – Product Description 117
IMMray Rheumatoid Arthritis Assay – Product Status 117
IMMray Rheumatoid Arthritis Assay – Product Description 117
IMMray SLE-d Test – Product Status 118
IMMray SLE-d Test – Product Description 118
IMMray Systemic Vasculitis Assay – Product Status 118
IMMray Systemic Vasculitis Assay – Product Description 119
ImmusanT Inc Pipeline Products & Ongoing Clinical Trials Overview 120
Nexvax2 Companion Diagnostic Test – Product Status 120
Nexvax2 Companion Diagnostic Test – Product Description 120
Serology Test – Celiac Disease – Product Status 121
Serology Test – Celiac Disease – Product Description 121
Innobiochips Pipeline Products & Ongoing Clinical Trials Overview 122
Diagnostic Assay – Autoimmune Diseases – Product Status 122
Diagnostic Assay – Autoimmune Diseases – Product Description 122
Inova Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 123
BIO-FLASH – ANA Profile Assay – Product Status 123
BIO-FLASH – ANA Profile Assay – Product Description 123
BIO-FLASH – Chromatin Assay – Product Status 124
BIO-FLASH – Chromatin Assay – Product Description 124
BIO-FLASH – F Actin Assay – Product Status 124
BIO-FLASH – F Actin Assay – Product Description 125
BIO-FLASH – gp210 Assay – Product Status 125
BIO-FLASH – gp210 Assay – Product Description 125
BIO-FLASH – sp100 Assay – Product Status 126
BIO-FLASH – sp100 Assay – Product Description 126
Biomarker Test – Autoimmune Liver Disease – Product Status 126
Biomarker Test – Autoimmune Liver Disease – Product Description 127
InterVenn BioSciences Pipeline Products & Ongoing Clinical Trials Overview 128
Treatment Monitoring Panel – Autoimmune Diseases – Product Status 128
Treatment Monitoring Panel – Autoimmune Diseases – Product Description 128
Iron Horse Diagnostics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 129
ALS Biologic Prognostic Test – Product Status 129
ALS Biologic Prognostic Test – Product Description 129
Multiple Sclerosis Disease Activity/Treatment Response Test – Product Status 130
Multiple Sclerosis Disease Activity/Treatment Response Test – Product Description 130
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 131
Biomarker Based Diagnostic Assay – Multiple Sclerosis – Product Status 131
Biomarker Based Diagnostic Assay – Multiple Sclerosis – Product Description 131
Lankenau Institute for Medical Research Pipeline Products & Ongoing Clinical Trials Overview 132
ANRE Assay – Product Status 132
ANRE Assay – Product Description 132
LineaGen Inc Pipeline Products & Ongoing Clinical Trials Overview 133
Diagnostic Test – Multiple Sclerosis – Product Status 133
Diagnostic Test – Multiple Sclerosis – Product Description 133
Lophius Biosciences GmbH Pipeline Products & Ongoing Clinical Trials Overview 134
T-Track Multiple Sclerosis – Product Status 134
T-Track Multiple Sclerosis – Product Description 134
Louisville Bioscience Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 135
Lupus pT Autoimmune Test – Product Status 135
Lupus pT Autoimmune Test – Product Description 135
Multiple Sclerosis pT Autoimmune Test – Product Status 136
Multiple Sclerosis pT Autoimmune Test – Product Description 136
Polymyositis pT Autoimmune Test – Product Status 136
Polymyositis pT Autoimmune Test – Product Description 137
Scleroderma pT Autoimmune Test – Product Status 137
Scleroderma pT Autoimmune Test – Product Description 137
Max Delbruck Center for Molecular Medicine Pipeline Products & Ongoing Clinical Trials Overview 138
Diagnostic Test – Raynaud’s Syndrome – Product Status 138
Diagnostic Test – Raynaud’s Syndrome – Product Description 138
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 139
Antibody Test – Multiple Sclerosis – Product Status 139
Antibody Test – Multiple Sclerosis – Product Description 139
Medica Corp Pipeline Products & Ongoing Clinical Trials Overview 140
EasyRA Analyzer – IgA Assay – Product Status 140
EasyRA Analyzer – IgA Assay – Product Description 140
EasyRA Analyzer – IgG Assay – Product Status 141
EasyRA Analyzer – IgG Assay – Product Description 141
EasyRA Analyzer – IgM Assay – Product Status 141
EasyRA Analyzer – IgM Assay – Product Description 142
Medical & Biological Laboratories Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 143
Anti-CADM-140 Autoantibodies Detection Kit – Product Status 143
Anti-CADM-140 Autoantibodies Detection Kit – Product Description 143
Metabiomics Corporation Pipeline Products & Ongoing Clinical Trials Overview 144
Microbiome Biomarker Test – Autoimmune Diseases – Product Status 144
Microbiome Biomarker Test – Autoimmune Diseases – Product Description 144
Microbiome Biomarker Test – Colitis – Product Status 145
Microbiome Biomarker Test – Colitis – Product Description 145
Microbiome Biomarker Test – Crohn’s Disease – Product Status 145
Microbiome Biomarker Test – Crohn’s Disease – Product Description 146
Metabolomic Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 147
CeliacDx – Product Status 147
CeliacDx – Product Description 147
ColitisDx – Product Status 148
ColitisDx – Product Description 148
CrohnsDx – Product Status 148
CrohnsDx – Product Description 149
IBDDx – Product Status 149
IBDDx – Product Description 149
Metanomics Health GmbH Pipeline Products & Ongoing Clinical Trials Overview 150
Diagnostic Assay – Multiple Sclerosis – Product Status 150
Diagnostic Assay – Multiple Sclerosis – Product Description 150
MSDx Inc Pipeline Products & Ongoing Clinical Trials Overview 151
WINDOW INTO THE BRAIN Test Panel – Multiple Sclerosis – Product Status 151
WINDOW INTO THE BRAIN Test Panel – Multiple Sclerosis – Product Description 151
Myriad Genetics Inc Pipeline Products & Ongoing Clinical Trials Overview 152
Diagnostic Assay – Juvenile Idiopathic Rheumatoid Arthritis – Product Status 152
Diagnostic Assay – Juvenile Idiopathic Rheumatoid Arthritis – Product Description 152
Nanosphere Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 153
Ultra-Sensitive Protein Assay – Autoimmune Disease – Product Status 153
Ultra-Sensitive Protein Assay – Autoimmune Disease – Product Description 153
Neuro Vigor LLC Pipeline Products & Ongoing Clinical Trials Overview 154
Acrolein Based Companion Diagnostic Assay – Multiple Sclerosis – Product Status 154
Acrolein Based Companion Diagnostic Assay – Multiple Sclerosis – Product Description 154
NoToPharm srl Pipeline Products & Ongoing Clinical Trials Overview 155
Diagnostic Assay – Dermatomyositis – Product Status 155
Diagnostic Assay – Dermatomyositis – Product Description 155
Diagnostic Assay – Mixed Connectivitis – Product Status 156
Diagnostic Assay – Mixed Connectivitis – Product Description 156
Diagnostic Assay – Polimyositis – Product Status 156
Diagnostic Assay – Polimyositis – Product Description 157
Diagnostic Assay – Sjogren Syndrome – Product Status 157
Diagnostic Assay – Sjogren Syndrome – Product Description 157
Diagnostic Assay – Systemic Lupus Erythematosus – Product Status 158
Diagnostic Assay – Systemic Lupus Erythematosus – Product Description 158
Diagnostic Assay – Systemic Sclerosis – Product Status 158
Diagnostic Assay – Systemic Sclerosis – Product Description 159
IFI16Ab Based Prognostic Assay – Product Status 159
IFI16Ab Based Prognostic Assay – Product Description 159
Numares AG Pipeline Products & Ongoing Clinical Trials Overview 160
Axinon – Multiple Sclerosis – Product Status 160
Axinon – Multiple Sclerosis – Product Description 160
OmniBiome Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 161
Pediatric Diagnostic Test – Autoimmune Disease – Product Status 161
Pediatric Diagnostic Test – Autoimmune Disease – Product Description 161
One Way Liver SL Pipeline Products & Ongoing Clinical Trials Overview 162
Diagnostic Test – Multiple Sclerosis – Product Status 162
Diagnostic Test – Multiple Sclerosis – Product Description 162
OPKO Health Inc Pipeline Products & Ongoing Clinical Trials Overview 163
Diagnostic Test – Multiple Sclerosis – Product Status 163
Diagnostic Test – Multiple Sclerosis – Product Description 163
Op-T-Mune Inc Pipeline Products & Ongoing Clinical Trials Overview 164
Screening Test – Multiple Sclerosis – Product Status 164
Screening Test – Multiple Sclerosis – Product Description 164
Oxford Biodynamics Plc Pipeline Products & Ongoing Clinical Trials Overview 165
EpiSwitch T2DM Prognostic Test – Product Status 165
EpiSwitch T2DM Prognostic Test – Product Description 165
Oxford Gene Technology Ltd Pipeline Products & Ongoing Clinical Trials Overview 166
OGT SLE Test – Product Status 166
OGT SLE Test – Product Description 166
Oxford Immunotec Ltd Pipeline Products & Ongoing Clinical Trials Overview 167
Stratokine – Product Status 167
Stratokine – Product Description 167
Pediatric Bioscience LLC Pipeline Products & Ongoing Clinical Trials Overview 168
MAR Test – Product Status 168
MAR Test – Product Description 168
Penn State College of Medicine Pipeline Products & Ongoing Clinical Trials Overview 169
Multiple Sclerosis Biomarker Test – Product Status 169
Multiple Sclerosis Biomarker Test – Product Description 169
Progentec Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 170
aiMS DX Disease Activity Test – Multiple Sclerosis – Product Status 170
aiMS DX Disease Activity Test – Multiple Sclerosis – Product Description 170
aiMS DX Interferon Treatment Response – Multiple Sclerosis – Product Status 171
aiMS DX Interferon Treatment Response – Multiple Sclerosis – Product Description 171
aiMS DX NMO Distinction – Neuromyelitis Optica – Product Status 171
aiMS DX NMO Distinction – Neuromyelitis Optica – Product Description 172
aiMS DX Relapse Prediction Test – Multiple Sclerosis – Product Status 172
aiMS DX Relapse Prediction Test – Multiple Sclerosis – Product Description 172
aiSLE DX Classification Test – Product Status 173
aiSLE DX Classification Test – Product Description 173
aiSLE DX Disease Activity Test – Product Status 173
aiSLE DX Disease Activity Test – Product Description 174
Biologic Biomarker – Crohn’s Disease / Ulcerative Colitis – Product Status 174
Biologic Biomarker – Crohn’s Disease / Ulcerative Colitis – Product Description 174
Diagnostic Test – Sjogren’s Syndrome – Product Status 175
Diagnostic Test – Sjogren’s Syndrome – Product Description 175
Prometheus Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 176
Companion Diagnostic Assay – Ulcerative Colitis – Product Status 176
Companion Diagnostic Assay – Ulcerative Colitis – Product Description 176
PRA052 Companion Diagnostic Assay – Product Status 177
PRA052 Companion Diagnostic Assay – Product Description 177
Prometheus Biosciences Inc – Ongoing Clinical Trials Overview 178
Companion Diagnostic Assay – Ulcerative Colitis – A Phase 2, Multi-center, Double-blind, Placebo-controlled Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of Induction Therapy with PRA023 in Subjects with Moderately to Severely Active Ulcerative Colitis 179
PRA052 Companion Diagnostic Assay – A Phase I, Double-Blind, Placebo-Controlled, Safety, Tolerability and Pharmacokinetics Study of PRA052 in Healthy Volunteers 180
Protagen AG Pipeline Products & Ongoing Clinical Trials Overview 181
Diagnostic Assay – Ankylosing Spondylitis – Product Status 181
Diagnostic Assay – Ankylosing Spondylitis – Product Description 181
Diagnostic Assay – Juvenile Idiopathic Arthritis – Product Status 182
Diagnostic Assay – Juvenile Idiopathic Arthritis – Product Description 182
Diagnostic Assay – Multiple Sclerosis – Product Status 182
Diagnostic Assay – Multiple Sclerosis – Product Description 183
Diagnostic Assay – Neuromyelitis Optica – Product Status 183
Diagnostic Assay – Neuromyelitis Optica – Product Description 183
Multilisa SLE – Product Status 184
Multilisa SLE – Product Description 184
Multiplex Assay – Autoimmune Diseases – Product Status 184
Multiplex Assay – Autoimmune Diseases – Product Description 185
NavigAID – Sjogren’s Syndrome – Product Status 185
NavigAID – Sjogren’s Syndrome – Product Description 185
ProteinLogic Ltd Pipeline Products & Ongoing Clinical Trials Overview 186
ImmiPrint Diagnostic Test – Autoimmune Diseases – Product Status 186
ImmiPrint Diagnostic Test – Autoimmune Diseases – Product Description 186
Psyros Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 187
Psyros – Autoimmune Disease – Product Status 187
Psyros – Autoimmune Disease – Product Description 187
Quanterix Corp Pipeline Products & Ongoing Clinical Trials Overview 188
ADVIA Centaur sNFL Assay – Multiple Sclerosis – Product Status 188
ADVIA Centaur sNFL Assay – Multiple Sclerosis – Product Description 188
Digital ELISA – Crohn’s Disease – Product Status 189
Digital ELISA – Crohn’s Disease – Product Description 189
Rambam Health Care Campus Pipeline Products & Ongoing Clinical Trials Overview 190
Molecular Diagnostic Test – Multiple Sclerosis – Product Status 190
Molecular Diagnostic Test – Multiple Sclerosis – Product Description 190
Rappaport Family Institute for Research in the Medical Science Pipeline Products & Ongoing Clinical Trials Overview 191
Biomarker Test – Multiple Sclerosis – Product Status 191
Biomarker Test – Multiple Sclerosis – Product Description 191
Revvity Inc Pipeline Products & Ongoing Clinical Trials Overview 192
Accentis – Autoimmune Disorders – Product Status 192
Accentis – Autoimmune Disorders – Product Description 192
Roche Diagnostics Pipeline Products & Ongoing Clinical Trials Overview 193
Roche Elecsys NfL Test – Product Status 193
Roche Elecsys NfL Test – Product Description 194
Roche Diagnostics International Ltd Pipeline Products & Ongoing Clinical Trials Overview 195
Anti-IL17 Mab Companion Diagnostic Assay – Product Status 195
Anti-IL17 Mab Companion Diagnostic Assay – Product Description 195
Companion Diagnostic Test – Ocrelizumab – Product Status 196
Companion Diagnostic Test – Ocrelizumab – Product Description 196
IFN-Induced Genes Companion Diagnostic Test – Product Status 196
IFN-Induced Genes Companion Diagnostic Test – Product Description 197
Integrated Modular Analytics – IL-6 Test – Product Status 197
Integrated Modular Analytics – IL-6 Test – Product Description 197
Royal College of Surgeons Ireland Pipeline Products & Ongoing Clinical Trials Overview 198
Diagnostic Biomarker Assay – Maturity-Onset Of Diabetes Of The Young – Product Status 198
Diagnostic Biomarker Assay – Maturity-Onset Of Diabetes Of The Young – Product Description 198
Seattle Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 199
Biomarker Test – Juvenile Systemic Sclerosis – Product Status 199
Biomarker Test – Juvenile Systemic Sclerosis – Product Description 199
Genetic Test – Kawasaki Disease – Product Status 200
Genetic Test – Kawasaki Disease – Product Description 200
Sengenics Corp Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 201
Companion Diagnostic Test – Autoimmune Disease Drug 1 – Product Status 201
Companion Diagnostic Test – Autoimmune Disease Drug 1 – Product Description 201
Companion Diagnostic Test – Autoimmune Disease Drug 2 – Product Status 202
Companion Diagnostic Test – Autoimmune Disease Drug 2 – Product Description 202
Diagnostic Assay – Rheumatoid Arthritis – Product Status 202
Diagnostic Assay – Rheumatoid Arthritis – Product Description 203
Diagnostic Assay – Sjogren Syndrome – Product Status 203
Diagnostic Assay – Sjogren Syndrome – Product Description 203
Diagnostic Assay – Systemic Lupus Erythematosus – Product Status 204
Diagnostic Assay – Systemic Lupus Erythematosus – Product Description 204
Sequenom Inc Pipeline Products & Ongoing Clinical Trials Overview 205
AttoSense Lupus Panel – Product Status 205
AttoSense Lupus Panel – Product Description 205
Shuwen Biotech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 206
Diagnostic Test – Autoimmune Diseases – Product Status 206
Diagnostic Test – Autoimmune Diseases – Product Description 206
SomaLogic Inc Pipeline Products & Ongoing Clinical Trials Overview 207
SomaSignal Test – Multiple Sclerosis – Product Status 207
SomaSignal Test – Multiple Sclerosis – Product Description 207
Southern Illinois University Carbondale Pipeline Products & Ongoing Clinical Trials Overview 208
Diagnostic Test – Crohn’s Disease – Product Status 208
Diagnostic Test – Crohn’s Disease – Product Description 208
Diagnostic Test – Ulcerative Colitis – Product Status 209
Diagnostic Test – Ulcerative Colitis – Product Description 209
Spanish National Research Council Pipeline Products & Ongoing Clinical Trials Overview 210
Diagnostic Assay – Rheumatoid Arthritis – Product Status 210
Diagnostic Assay – Rheumatoid Arthritis – Product Description 210
SQI Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 211
ANA Ig_plex 11-Plex Assay – Product Status 211
ANA Ig_plex 11-Plex Assay – Product Description 212
Autoimmune Thyroid Panel 3-Plex Assay – Product Status 212
Autoimmune Thyroid Panel 3-Plex Assay – Product Description 212
Ig_plex 9-Plex – Product Status 213
Ig_plex 9-Plex – Product Description 213
Ig_PLEX Lupus Panel Assay – Product Status 213
Ig_PLEX Lupus Panel Assay – Product Description 214
IgX PLEX Celiac Panel 6-Plex Assay – Product Status 214
IgX PLEX Celiac Panel 6-Plex Assay – Product Description 214
Vasculitis Ig_PLEX 3-Plex – Product Status 215
Vasculitis Ig_PLEX 3-Plex – Product Description 215
Statens Serum Institut Pipeline Products & Ongoing Clinical Trials Overview 216
Diagnostic Test – Systemic Autoimmune Disease – Product Status 216
Diagnostic Test – Systemic Autoimmune Disease – Product Description 216
Sugentech Inc Pipeline Products & Ongoing Clinical Trials Overview 217
Diagnostic Test – Autoimmune Disease – Product Status 217
Diagnostic Test – Autoimmune Disease – Product Description 217
Svar Life Science AB Pipeline Products & Ongoing Clinical Trials Overview 218
Diagnostic Test – Autoimmune Disorders – Product Status 218
Diagnostic Test – Autoimmune Disorders – Product Description 218
TBG Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 219
TBG Kit – Autoimmune Disease – Product Status 219
TBG Kit – Autoimmune Disease – Product Description 219
Tel Aviv University Pipeline Products & Ongoing Clinical Trials Overview 220
Diagnostic Kit – Chronic Kidney Disease – Product Status 220
Diagnostic Kit – Chron
![]()